The mission of the Melis lab is to understand neurobiological underpinnings (e.g., adaptations of both mesocorticolimbic dopamine pathway and endocannabinoid system) of susceptibility and resilience to neuropsychiatric disorders.
I also aim to mentor the next generation of neuroscientists in an inspiring, collaborative, inclusive and safe environment (see Team).
“Only those who will risk going too far
can possibly find out how far one can go.”
T.S. Eliot
Whether or not vulnerability risk factors are innate (i.e., temperaments, sex) or induced by early life adverse events (e.g. drug exposure, neglect, adverse social events) will be thoroughly investigated via an integrative approach including cellular physiology, neuroanatomy, molecular and behavioral analyses.
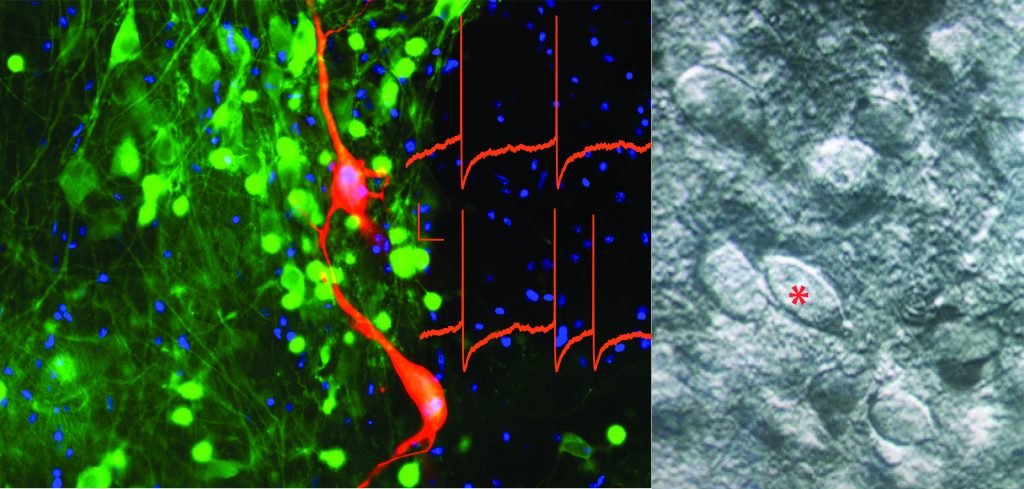
From left: Dopamine (TH, green) neurons backfilled by including biocytin (red) in the recording pipette during whole-cell patch-clamp recording. Current clamp traces of dopamine cell spontaneous activity (in red overlaid) and, on the right, DIC image of a putative dopamine neuron (*).
This is based upon my personal interest in neuropsychiatric disorders, particularly those mirroring affective imbalances within the brain. Affects are essential for well-being, and once primary physiological needs and safety are satisfied, interpersonal interaction and acceptance represent needs that have to be met, and drive motivation. In fact, they act as natural reinforcers, and are important for social and cognitive development.
The mesocorticolimbic dopamine system, key component of brain reward circuitry, processes value-related signals, and is considered an important circuit for affective functioning. Modulation of pathways determining the strength and direction of synaptic plasticity of such a system is, therefore, crucial in regulating experience-dependent behavioral changes, and viceversa. In particular, the individual response to early life adverse experiences depends upon an organism’s innate biological make-up.
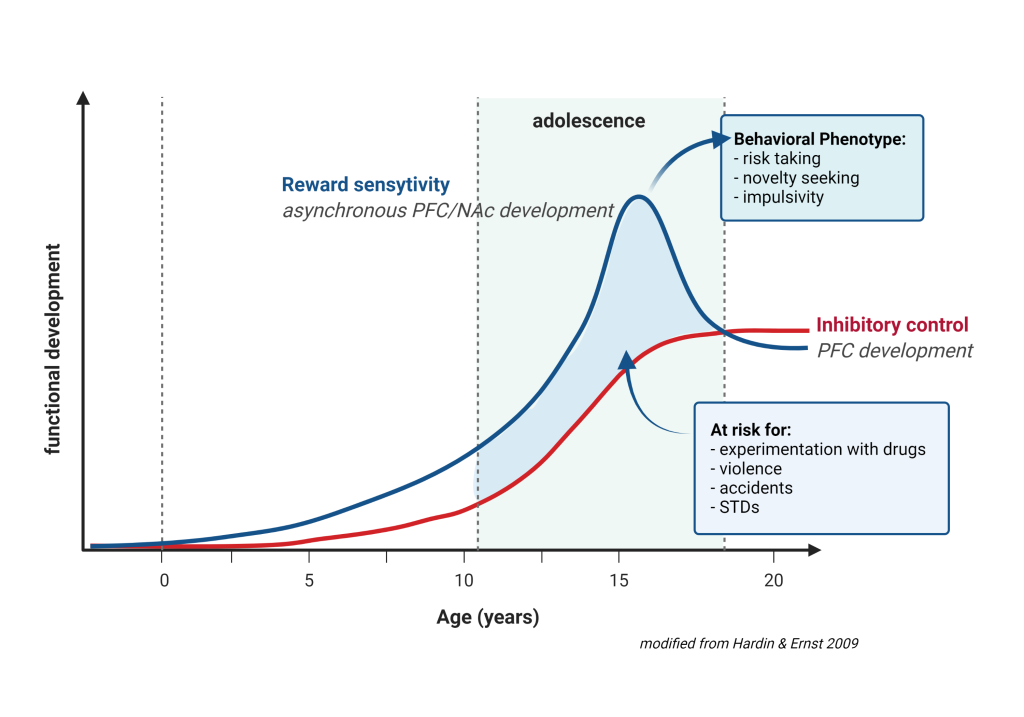
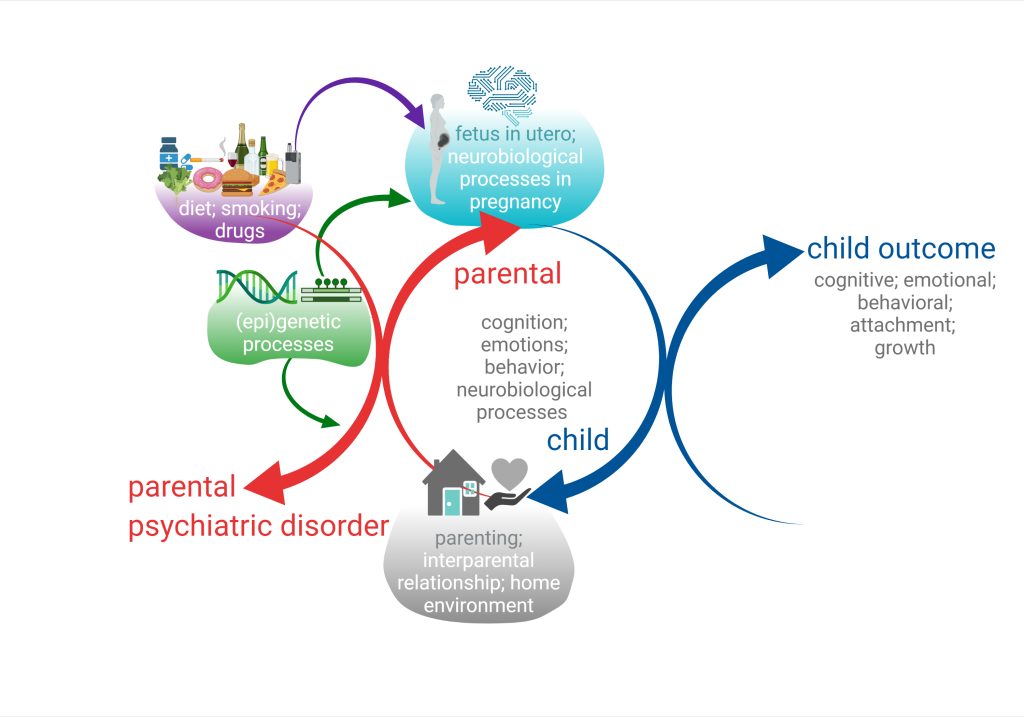
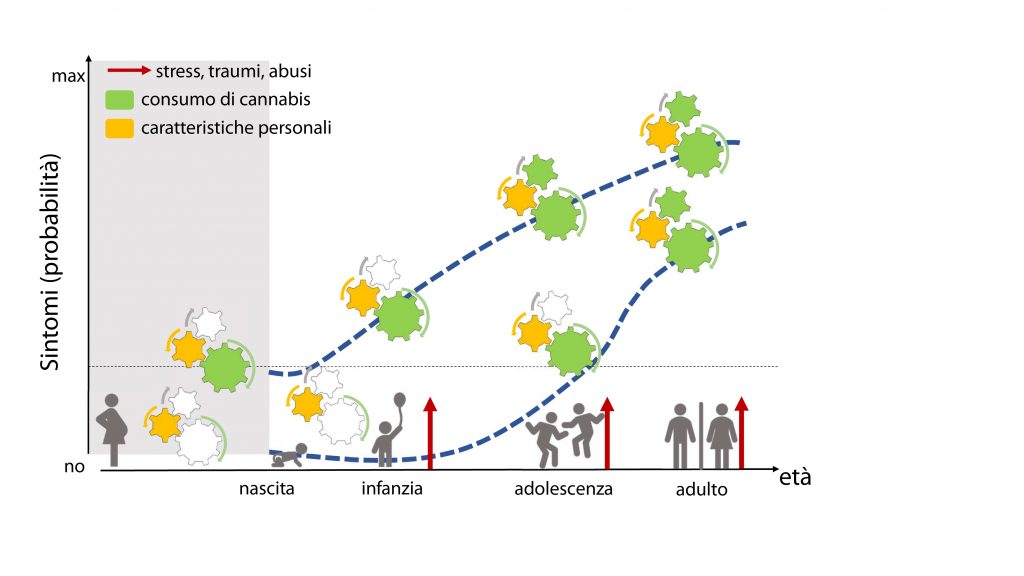
We began to address this issue by examining those associations between parental disorders/behavior and offspring outcomes from fetal development to adolescence (Melis et al. 2013, 2014; Frau et al 2019a; Frau et al. 2019b; Sagheddu et al. 2021; Traccis et al. 2021; Serra et al, 2023). We aim to specifically assess the role of mediating variables, underlying the links between parent psychopathology and offspring outcome, and possible moderators, which may change the strength of any given association, and focus on factors that are potentially modifiable (see diagrams below).
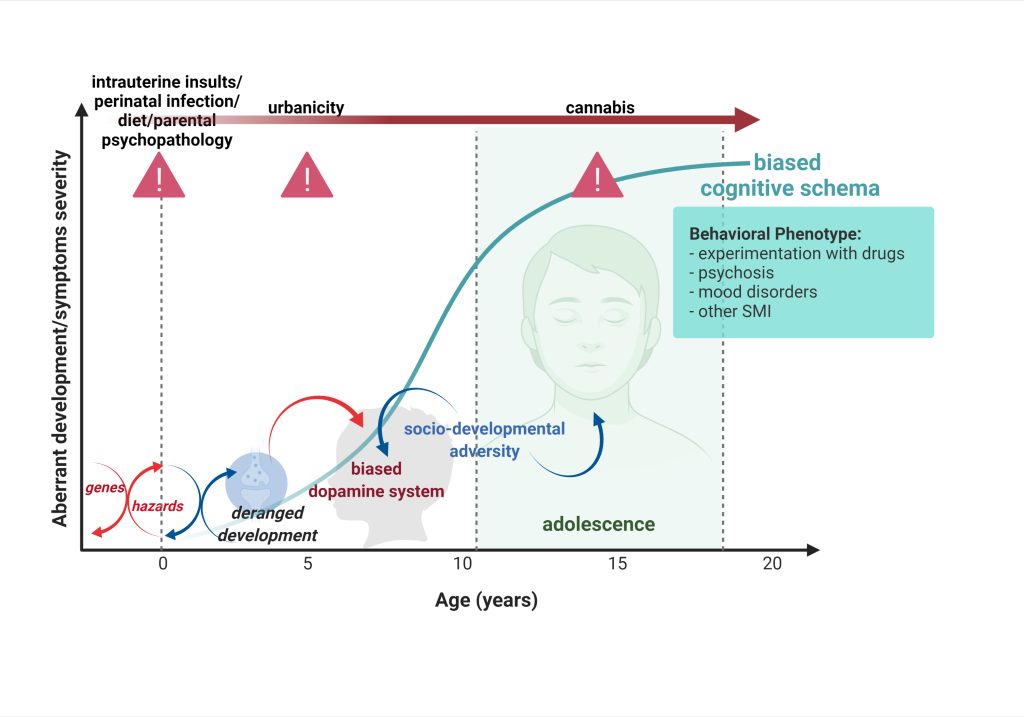
The findings will enable us to develop new pharmacological strategies for the prevention and the treatment of aberrant affective functioning, especially by investigating the mechanisms of resilience to rescue deranged developmental trajectories.
The Melis’ lab develops rodent models for aberrant dopamine and endocannabinoid signaling to further understand their interplay in brain health and disease.

The Melis lab is a Neuropsychopharmacology lab located at the University of Cagliari (Italy) in the Department of Biomedical Sciences, Division of Neuroscience and Clinical Pharmacology.
Lab address is at the University Campus in Monserrato (Building C, ground floor, rooms T5 and T7).
Miriam is Professor of Pharmacology.
Her office is located at the University Campus in Monserrato:
Building C, floor 1, room #15
Tel: +39 070 675 4322
Lab tel: +39 070675 4340
FAX +39 070 6754320